Development of multi-allergen site and first production of egg and milk
challenge meals
Press Release
20 th May 2025 marks another key milestone for Reacta Healthcare, as the first
production run of milk and egg challenge meals commence on site for the first time.
These first orders will be shipped overseas for a phase III and a phase II clinical trial
investigating novel food allergy treatments.
The importance of considering multiple food allergies in both diagnosis and
treatment has become increasingly evident. Many individuals suffering from food
allergies often experience reactions to more than one allergen, making it crucial for
healthcare providers to adopt a holistic approach. . Clinicians can focus care on the
needs of the individual which improves patient outcomes and enhances the quality of
life for those affected by food allergies.
The addition of egg and milk challenge meals to the existing peanut products at
Reacta Healthcare highlights the significance of addressing multiple allergens in
clinical trials. These products are designed to meet the needs of food allergy
research and clinical communities, ensuring safety, quality, and efficacy across
various allergens. The standardised development of these new allergen products
allows for easy expansion of the product portfolio with further allergens.
Full development and manufacture of the new products has been undertaken at the
Medicines & Healthcare products Regulatory Agency (MHRA) licensed site in
Deeside, UK. The products were designed using the Quality by Design approach
which is a systematic and strategic approach employed in pharmaceuticals. This
ensures the consistent delivery of high-quality products with a pre-determined
objective to ensure that the product is fit for intended use. A standardised approach
across multiple allergens has been implemented to ensure the safety, quality, and
efficacy of the challenge meals.
The challenge meals comply with client requirements for allergen identification,
standardisation, and cGMP manufacturing.
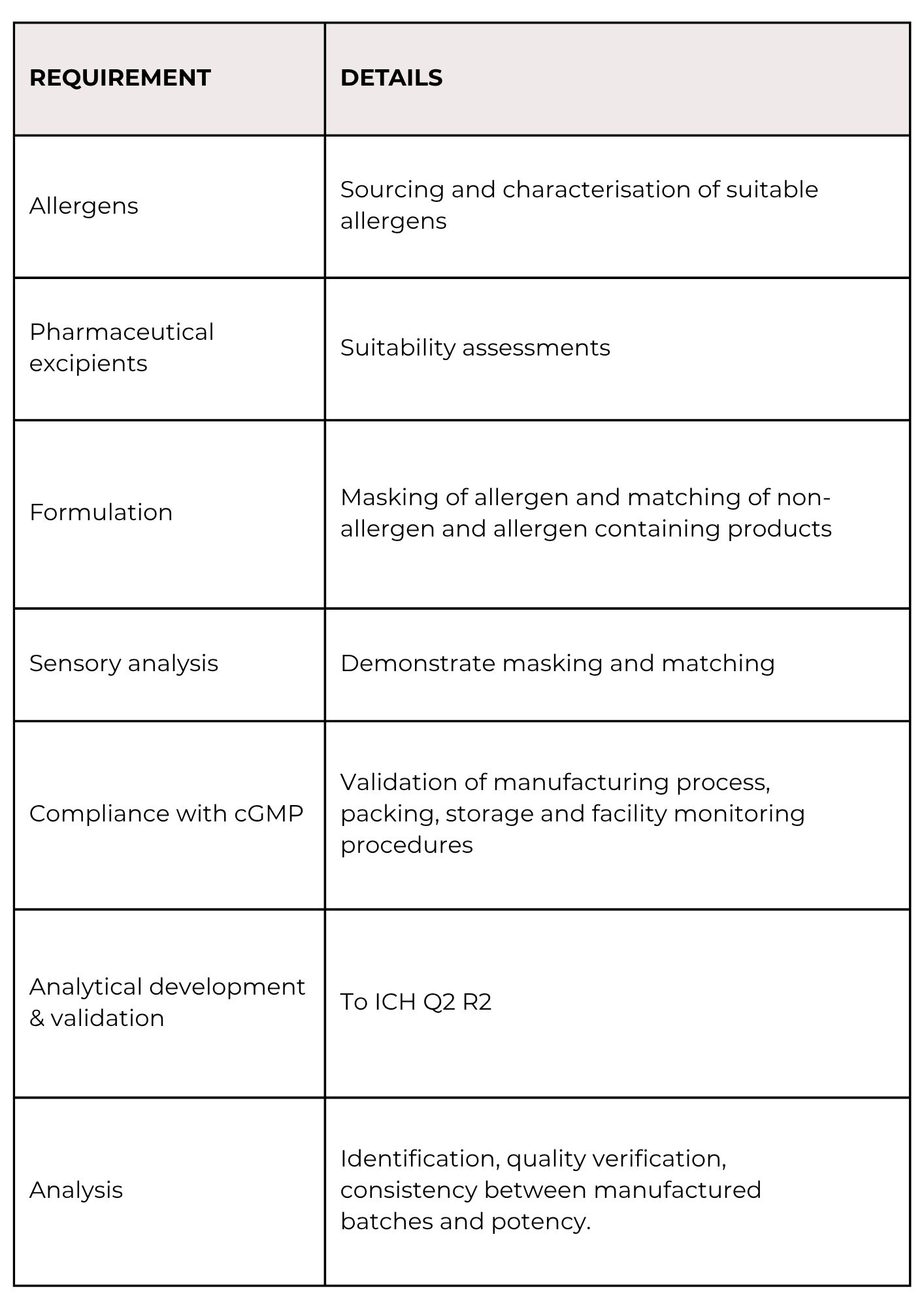
Products come complete with dossier suitable for submission of IND Module 3.
Successful submissions of these dossiers have been made to various health
agencies including FDA and EMA on numerous occasions since 2017.
These challenge meals are suitable for supply to phase I, II and III clinical trials for
use in double-blind placebo-controlled oral food challenges. With the addition of 2
more allergens Reacta is ideally placed to supply various challenge meals to clinical
trials seeking an ‘antigen agnostic’ indication for their respective therapy. The multi-
allergen site is now set up to seamlessly develop further allergen challenge meals as
demand increases across the USA ‘Top 9’ allergens: milk, eggs, peanuts, fish, crustacean shellfish, tree nuts, wheat, soybeans, and sesame.
The company is eagerly exploring new opportunities to leverage these innovative
products to revolutionise the food allergy research and clinical community. Reacta is
exploring innovative new solutions, using advanced technology and research to
improve food allergy diagnosis and management.
About Reacta Healthcare
Reacta Healthcare, established in 2013, operates from a pharmaceutical
manufacturing facility in the UK. The MHRA licensed facility is licenced to manufacture oral challenge meals, for use in clinical trials, as non-investigational medicinal products (NIMPs)/auxiliary medicinal products (AxMPs). The Reacta Healthcare challenge meals are currently used to diagnose and monitor food allergy in numerous global therapeutic trials. Patents for the challenge meals have been granted in a number of countries.
